Approaching Quality with Patients in Mind
Our uncompromising commitment to quality and compliance begins with research and development. And it covers each stage of the drug development and commercialization process, from the procurement of raw materials, to the approval of our product submissions by regulatory authorities around the world and commercial distribution.
We validate and continually monitor our manufacturing processes to ensure they perform as expected. Each of our products is tested to confirm compliance to Zahra’s strict quality specifications and compliance standards. As a result, we have an enviable record of delivering the highest quality products and thoroughly exceed regulatory standards.
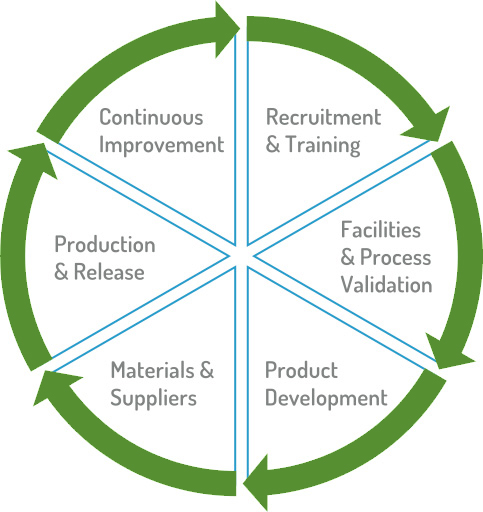
Quality in Recruitment and Training
Our people are our greatest asset, and their dedication creates an unmatched level of client service that establishes our company as the leader in our industry. Zahra Pharma leadership has dedicated recruitment staff who identify individuals with the strengths necessary to excel in this specialized industry. We also provide our people with a formalized training and the tools they need to provide quality products to your patients.
Quality in Facilities and Process Validation
Our facilities are designed for compliance with all regulations, and we continuously strive to improve workflow processes. Our customers are welcome to perform audits to see firsthand how we build in quality across our facilities. All cleanrooms and equipment are robustly qualified and routinely calibrated.
Quality in Product Development
We constantly uphold our core value of excellence in providing generic & specialty medications that are backed by quality standards, safety, integrity, accountability and outstanding service. We work with our customers to identify those products that meet their needs. Product development involves extensive evaluation prior to commercial production and it also includes stability testing to establish an appropriate Beyond Use Dating (BUD) while ensuring that labeling on finished products meets all applicable standards.
Quality in Materials and Suppliers
Quality products start with quality raw materials. The raw materials used by Zahra Pharma are in compliance with the latest regulations and produced in compliant facilities. Zahra Pharma has robust Vendor Qualification, Supplier Standards, and acceptance criteria for Raw Materials that are in compliance with cGMP standards.
Quality in Production and Release
Finished products are then held in quarantine until all release testing is complete. The independent Quality Unit dispositions all batches after review of all associated batch and test documentation.


USA I ETHIOPIA I DJIBOUTI
7000 Central Pkwy, Suite 960
Atlanta, GA 30328
